Cruise Day 20
Speed 1 knots (kts) (on station)
Course 252° (WSW)
Location Makarov Basin, approx. 380nm S. of the North Pole
Depth 3035 m
GO DEEPER DISCUSSION: (see previous journal for the questions.)
One of the biggest challenges of using satellite imagery to predict ice conditions is the presence of clouds and fog, which obscure clear views of the ice surface for visible satellites. RadarA method of estimating the distance or travel speed of an object by bouncing high frequency signals off the object and measuring the reflected signal. satellites can look for ice through clouds, but it isn’t always easy to determine ice thickness as the radar doesn’t penetrate the ice. When it is hard to accurately characterize the ice, estimated boundaries between types of ice are mapped.
TODAY’S JOURNAL:
We’re nearly done with the full sampling station we began around noon yesterday. The heavier ice makes things sometimes go a little slower as the crew tries to keep an open pool of water astern and at the starboard A-frame areas to get the gear in and out safely. Crew members take shifts with long, spiked ice poles to push away chunks of ice and the propellers can be used to create a gentle current for keeping the water open or to provide a stronger blast of propeller wash to push ice to the back of the “pond.” It is pretty neat to gaze down into the clear blue sea and contemplate nearly two miles of water separating us on the ship from the ocean floor.
When the Go Flo bottles come back from a GEOTRACES cast, they go into a clean van (read the Precious Water Journal) where the samples are divvied up in a process called sub-sampling. Different groups aboard the ship get their allotment of water, and other samples are stabilized and crated up to be shipped back to scientists at their home labs. One of the groups doing water analysis aboard Healy on this cruise is Team Mercury. Carl Lamborg, Kaitlin Bowman, and Alison Agather are aboard to measure mercury in the GEOTRACES water samples, and I visited with Kaitlin and Alison today in Team Mercury’s science van to see what they do.

Kaitlin is a Post-Doctoral Fellow at the University of California, Santa Cruz, and Alison is in the second year of her doctoral program at Wright State University, Ohio. Along with Carl they are looking to answer questions such as how mercury is distributed in the water column and how it behaves in marine systems. Natural sources such as volcanic emissions contribute to mercury in seawater. But humans also add mercury to the atmosphere through activities such as burning coal and by the production of various metals, and that mercury can later get deposited into the oceans. This human-contributed (anthropogenic) mercury is a concern due to its toxicity and the way that it bio-accumulates upwards through food webs. This means that there is a concentrating effect that starts with tiny amounts of the metal in organisms like phytoplankton or zooplankton. Let’s say that a fish like an arctic cod eats uncountable zillions of planktonic organisms in its life: It accumulates mercury that was contained in all of them. Then something like a ringed seal eats hundreds or thousands of arctic cod, further concentrating the mercury from them in its tissue. A polar bear living on seals then gets their accumulated mercury. For this reason, native Arctic humans who rely on subsistence hunting of seals, whales, and fish for much of their diet can accumulate levels of mercury in their bodies that are a serious concern.

Team Mercury looks at four forms of mercury in their water samples: Elemental mercury (Hg), dimethyl mercury, methyl mercury, and total mercury (the three forms named above and all other mercury-containing compounds.) They begin with 2-liter water samples, through which nitrogen is bubbled. The agitation produced by inert nitrogen bubbling releases gasseous elemental mercury and dimethyl mercury from the sample. These are captured using chemical traps; gold-coated sand for mercury and a sorbent called Bond Elut for dimethyl mercury. These traps are heated on an instrument board to re-vaporize the captured samples. The gasses from both mercury and dimethyl mercury traps goes through an atomic fluorescence detector, which bombards the gasses with UV light. The return fluorescent glow is measured and plotted on a curve. By calculating the area under the curve for each sample plot the scientists can precisely determine the minute amount of mercury and dimethyl mercury in each sample. (The units used for these measurements are femtomoles, or parts per quadrillion.) The trace amounts of mercury vapor run through the system are re-captured at the end of the process in a final chemical trap.
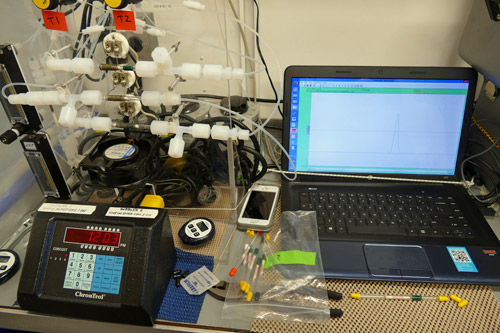
Methyl mercury isn’t released as a gas in the bubbling step, so after the first two types of mercury are extracted the water is treated chemically to convert the methyl mercury into a new form, gaseous ethyl-methyl mercury. It can then be bubbled through a trap and analyzed with the same technique used for elemental and dimethyl mercury.
Finally, for total mercury a water sample is chemically treated with an oxidizing agent which converts all elemental mercury and mercury compounds to mercury 2+ ions. Again, this will off-gas with the nitrogen bubbling technique, get captured in a gold-coated sand trap, and be quantified with an atomic fluorescence detector. Total mercury in seawater is still scarce, somewhere on the order of 1 picomolar (~1 part per trillion.)
Mercury analysis is subject to contamination, which is why the samples come from the clean GEOTRACES collection process. Dust and atmospheric mercury can be problematic, and other labs aboard the ship use mercury compounds in their analysis. The mercury van has a HEPA-filtered hood to provide a clean environment for their work. The amount of mercury that goes through their samples is minuscule, but other chemicals like acids used in the methyl mercury conversion present a hazard that is managed with the use of controlled dispensing devices, splash guards, safety goggles, gloves, and a lab coat.
Thanks for walking me though your world, Team Mercury!
GO DEEPER!
The Metric System has standardized unit prefixes including pico- and femto- that were used in this journal post. If we want to divide a base unit into tenths, deci- is used. For hundredths we use centi-. What prefixes continue this pattern through pico and femto to the smallest agreed-upon unit prefix in the Metric System?
Aloft Con web cam updated every hour
Healy Track
That's all for now.
Best- Bill


Comments