Science Update
Clarification: I misidentified Trinity Island as part of the South Shetland Island Chain. It turns out that Trinity Island is part of the Palmer ArchipelagoA chain of many islands.. Yesterday, we moved towards sampling stations in the Bransfield Straight, just south of the actual South Shetland Islands. We sampled seawater using both rosettes at sampling stations near Livingston Island. After sampling, we were able to enjoy the beautiful views of penguin-covered glaciers, mountains and bays of the island.


Today, we are traveling to Nelson Island and King George Island to collect seawater in three separate sample stations. This area is historically known for higher concentrations of iron, as compared to water in the Drake PassageStrait, connecting the Atlantic and Pacific oceans between Tierra del Fuego and the South Shetland Islands. Located about 100 mi (160 km) north of the Antarctic Peninsula, it is 600 mi (1,000 km) wide. and other areas of the Southern Ocean.
![Sampling locations for September 19th include bays and open water near Nelson Island and King George Island. Both islands are located to the right of the highlighted Greenswich Island. By Apcbg (Own work) [CC BY-SA 3.0 (http://creativecommons.org/licenses/by-sa/3.0) or GFDL (http://www.gnu.org/copyleft/fdl.html)], via Wikimedia Commons South Shetland Island map](/files/members/cara-pekarcik/images/greenwich-island-location-map.jpg)
Confessions of a Biologist
I will admit that before I left for this research cruise, I would have told you that the purpose of the cruise was to study diatoms. The biologist in me didn't want to admit that ocean chemistry was also a major focus of the research. It turns out that without knowledge of the ocean chemistry, the biological analyses wouldn't tell the entire story of the diatoms. The ocean chemistry available to diatoms, in this case the nutrients and trace metals, influence the productivity of the diatoms in this region. Without the availability of certain nutrients, diatoms and other phytoplankton are unable to photosynthesize and, therefore, unable to live.
High Nutrient, Low-Chlorophyll (HNLC)
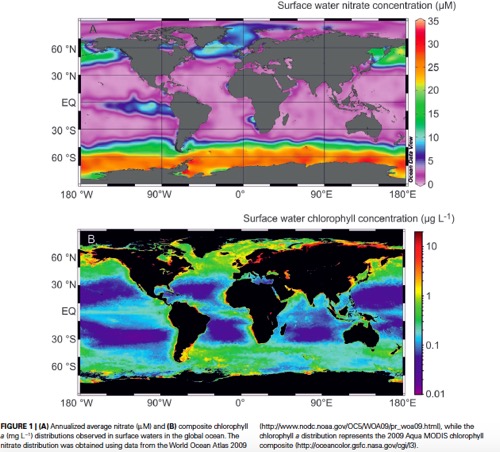
Historically, scientists recognized the Southern Ocean (along with the Sub-Arctic Pacific and the equatorial Pacific near Peru) as a low productivity area or an area with limited phytoplankton growth. They refer to the area as a HNLC (high nutrient, low-chlorophyll) region, meaning high levels of nutrients like nitrates and phosphates, but lower than expected amounts of chlorophyll used for photosynthesis. The graphics below show this HNLC concept. In the top graphic, high levels of nitrate are shown in oranges and reds. You can see that the Southern Ocean has extremely high nitrate levels (or HN). In the second graphic, the chlorophyll levels are shown (green being high). While the Southern Ocean does have areas of green indicating chlorophyll, the levels are much lower than expected (or LC) given the high amount of nitrogen in the area.
For many years, scientists believed that this low productivity in the Southern Ocean was caused by low light (light-dependency) or high amounts of zooplankton that feed on the phytoplankton in high amounts. In 1990, John H. Martin published a paper in the journal Paleogeography expressing his idea called the Iron Hypothesis. His paper claimed that iron was the limiting factor in phytoplankton productivity.
Iron
Iron is an important element for all living things. In animals, iron is required for oxygen to bind to the hemoglobin protein on our red blood cells. In plant material, iron is essential for a number of processes, including the production of chlorophyll, reducing nitrate into usable forms and fixing atmospheric nitrogen. These processes are necessary for plants and algae. Although iron is the 4th most abundant element in the Earth's crust, usable sources of iron are generally found in much lower concentrations in water. Iron can enter the water because of close vicinity to land, blowing sediments and remineralization by bacteria. In the Southern Ocean where almost everything is covered with ice or snow, there is no sediment (dust) to blow into the ocean. Iron from the continent can increase iron levels in locations close to shore, but not in the open ocean.
Continuing in Martin's Footsteps
John Martin hypothesized that the iron limitations in the HNLC areas had a major impact on the productivity of the phytoplankton. He completed experiments on water samples from the Southern Ocean to analyze the iron levels and conduct bottle experiments to see if additional iron caused higher productivity. These early experiments showed that when diatoms were provided with additional iron, their productivity increased.
In the time since John Martin proposed his Iron Hypothesis, chemists and biologist have worked together to learn more about the ocean chemistry and related biological processes in the Southern Ocean and throughout the world. The fact that iron is limited in the Southern Ocean is the basis for the research questions of this cruise. DiatomsDiatoms are one of the most common types of phytoplankton. Most diatoms are unicellular, although they can exist as colonies in the shape of filaments or ribbons. Diatom communities are a popular tool for monitoring environmental conditions, past and present, and are commonly used in studies of water quality. require iron for photosynthesis, but they live in an iron-deficient area. Even inland locations with higher iron concentrations like Nelson Island and King George Island still do not provide the diatoms with the amount of iron needed for their maximum productivity. So, how are diatoms able to survive in these waters? Do diatoms have special adaptations to allow them to increase productivity? Do diatoms use relationships with other organisms to aid their ability to obtain iron? These and other questions are used to choose sample stations and sample depths throughout the trip. In fact, we are even sampling at some of John Martin's original sampling stations during this research cruise. In future journals, I will try to explain some of the specific analyses that may allow scientists to better understand the Southern Ocean productivity.
Resources
Gledhill, M. and K. N. Buck (2012). The organic complexation of iron in the marine environment: a review. Frontiers in Microbiology: 3(69), 1-6.
Martin, J.H. (1990). Glacial-Interglacial CO2 Change: The Iron Hypothesis. Paleoceanography: 5(1), 1-13.
Morel, F.M.M, and N.M Price. (2003) The biogeochemical cycles of trace metals in the oceans. Science: 300(5621), 944-947.


Comments