Speed 0.5 knots
Course 306°
Location Drygalski Inlet (-64.73338333, -60.59362833)
Depth 684 meters
Right now we're exploring the open seas of the Larsen A. The Larsen B is still shut off from us by a row of icebergs that are pretty much impassible. The last couple of days we've been near the Drygalski GlacierA mass of ice that persists for many years and notably deforms and flows under the influence of gravity.. It was really warm (39° F) and we spent a lot of time on the bow (front) of the boat enjoying the views. This is a view of the Dryglaski GlacierA mass of ice that persists for many years and notably deforms and flows under the influence of gravity.. Look for the massive expanse of blue ice still attached to land. Pretty, huh?

CTDA research tool that is submerged in the water to measure conductivity (salinity), temperature, and depth.
When I first arrived on this boat, the research scientists used acronyms that sounded like gibberish. I had to make a list of them to keep track of what everyone was talking about. One of the most commonly used acronyms is CTDA research tool that is submerged in the water to measure conductivity (salinity), temperature, and depth.. Any guesses what it means?

This is the CTDA research tool that is submerged in the water to measure conductivity (salinity), temperature, and depth.. As the device is lowered to the bottom of the sea, the instruments at the bottom measure Conductivity, Temperature and Depth of the device, thus the abbreviation CTDA research tool that is submerged in the water to measure conductivity (salinity), temperature, and depth.. When it is brought back to the surface, the large gray canisters are used to collect water at different depths. Look carefully at the picture and you can see the top and bottom are forced open so that water can move through them. Once they hit a particular depth, they close to collect water from a particular depth.
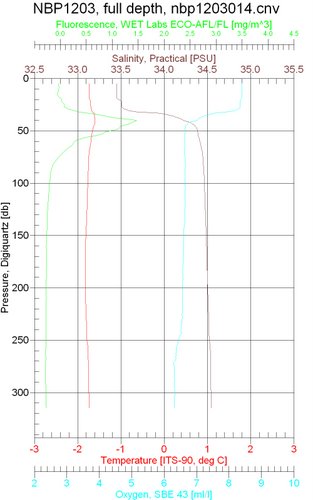
Below, you can see what we see as the CTDA research tool that is submerged in the water to measure conductivity (salinity), temperature, and depth. goes to the bottom. Hopefully my Marine Biology students (hi guys!) can see where the halocline (rapid change in salinity) is located in the water column. For those of you who are wondering, fluorescence often indicates an abundance of chlorophyll found in phytoplankton.
I climbed up to the uppermost part of the ship to get a view of the CTDA research tool that is submerged in the water to measure conductivity (salinity), temperature, and depth. being put into the water. A huge winch moves out over the water and then it is slowly lowered until it reaches about ten meters above the bottom.

Once the CTDA research tool that is submerged in the water to measure conductivity (salinity), temperature, and depth. is back on board, scientists rush to collect their samples. Some are collecting various biological organisms living at different depths, such as plankton, bacteria and viruses. Others are looking at the physical chemistry at different levels, like pH, salinity, and alkalinity. Regardless, there's always a mad dash for water as soon as it is back on the ship.

I've helped out with this process a lot and because I am a klutz, I always end up getting water on my shoes. Also my hands get pretty cold, because the water temperature is about 28.8° F (-1.7° C). Most of you might remember that water freezes at 32° F (0° C), so how come this water isn't frozen? The salt in the sea actually has a huge impact on the ability of water to freeze. Salt is made up of sodium (or potassium) ions that have a positive charge and chlorine molecules that have a negative charge. Water molecules are attracted to those ions and instead of forming bonds with other water molecules, they form bonds with the salt ions. This prevents the water molecules from forming their crystalline structures and water that has more salt in it has an even lower freezing point. Around here, the water generally has a freezing point around 28.9° F (-1.8° C), cold enough for my hands to be unhappy.


Comments