News Update
We are currently on a run headed east towards Banks Island with rosette CTDA research tool that is submerged in the water to measure conductivity (salinity), temperature, and depth. casts occurring about every 3 to 5 hours apart. It's all fairly routine at this point: rosette is cast, rosette is retrieved, samples are taken, samples are processed, samples are analyzed or stored, and repeat. Seita's equipment is working well busy storing data about the ice as we pass through it. Preparations for the first mooring operation are well underway with some "in deep" trouble shooting going on with a couple of pieces of new equipment.






A huge dam could stop water flowing from the Pacific to the Arctic Ocean. Very powerful turbines would pump cold water from the Arctic Ocean to the Pacific Ocean and sea level in the Arctic Ocean would decrease, initiating inflow of warm Atlantic waters to the Arctic via Fram Strait and the Barents Sea. This warm water would melt sea ice and climate would warm up. 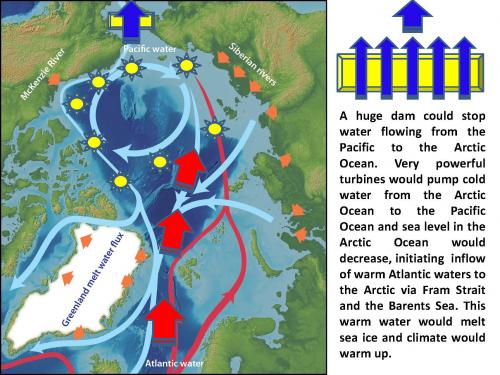
Chemistry Timeout
So let’s talk about carbon dioxide. When you open a bottle or can of carbonated beverage you are allowing carbon dioxide gas to come out of solution. This is because there is a pressure differential between the carbon dioxide in the liquid and carbon dioxide in the air. The pressure in the liquid is higher than the pressure in the air so the carbon dioxide moves from high to low. This is the tiny bubbles you see in the carbonated drink as it fizzes. This will go on until there is an equilibrium reached where the pressure inside the liquid is equal to the pressure of the air. It is at this point that the beverage is "flat"– as in no bubbles. So the question is how does the carbon dioxide get into the liquid in the first place, That is, in chemistry speak, how does carbon dioxide go into solution? There is an old adage in chemistry that "like dissolves like". This is a helpful way of predicting weather or not two chemicals will get together and form a solution. Polar molecules – those molecules with distinctly positive and negative ends – will dissolve other polar molecules. Non-polar molecules – molecules with an even distribution of positive and negative charge – will dissolve non-polar molecules. The problem with this is carbon dioxide (the solute) is non-polar and the water that it will dissolve in (the solvent) is polar. So why does carbon dioxide dissolve in water?
The answer is fairly straightforward. A good place to start however is at the particle level. The carbon dioxide is a linear molecule: Since there are lots of electrons around the oxygen atoms of both the water and carbon dioxide molecules and the hydrogen atom side of the water molecule is low electron density (so slightly positive) it is reasonable to assume that there must be some low level of attraction between the ends of the carbon dioxide and the hydrogen side of the water molecule. It is those very weak bonds that initiate the dissolving of carbon dioxide in water. That is where it all starts... but then it gets complicated but interesting. We'll talk about that in the near future.
Crew Member Focus
Cory Simms is one of the Stewards on the CCGS Louis St. Laurent. He makes his home in Bay Roberts, Newfoundland with his wife and 14 year old son. 
Sunday Semi-Science Fiction story
Geo-engineering methods to improve Arctic ClimateThe average weather over a particular region of the Earth. Climate originates in recurring weather phenomenon that result from specific types of atmospheric circulation. (warming 1) Our text below is based on some materials from archives of the Arctic and Antarctic Research Institute (AARI) recently provided by AARI deputy director, Dr. Igor Ashik. Some of this information was also published in the magazine "Popular Mechanics" (No. 1, January 2016). In the 1930s, the glaciologist Eugene Gernet suggested that the sea ice observed at that time in the Arctic was a product of the 20th century climate and therefore the sea ice conditions could be changed artificially to return the Arctic to the climate of the north of Eurasia in the early Miocene when cypress and magnolia trees covered lands of Scandinavia.
Later, this hypothesis was supported by the climatologist Mikhail Budyko (the future academician and author of the energy balance model, which became the basis of modern ideas about the climate and the greenhouse effect). In the late 1950s, Budyko advocated to reduce severances of Arctic climate by spraying a thin layer of soot above it. Absorbing sunlight, coal particles would help the total melting of ice at least during summer. The free of ice ocean water would also absorb solar radiation and delay sea ice formation in winter significantly extending duration of the Arctic navigation period.
An interesting idea for "radical climate improvement" was also introduced in 1959 by the geographer Peter Borisov who planned to block the Bering Strait with a dam equipped with huge water pumps. According to Borisov's calculations, daily pumping of 500 km3 of water from the Arctic Ocean to the Pacific Ocean should have resulted in the significant reduction of sea level in the Arctic Ocean compensated by the inflow of warm waters of Atlantic Ocean (Photo 8). The heat of the Atlantic water would melt Arctic ice and improve sea ice conditions and climate.
This project was widely discussed at that time. One of the problems was that the sea level of the Pacific Ocean is approximately 1 meter higher than the sea level of the Atlantic Ocean and this sea level difference between oceans is responsible for the Pacific Ocean water inflow to the Arctic via Bering Strait. The observed water transport via strait is approximately one Sverdrup (Sv, 1 Sv = 1 million m3/s) or 31,536 km3 per year. The Borisov’s dam even without pumping would stop this flow, change circulation of both the Arctic Ocean and the Bering Sea and would have significant impact on the climate of the Northern Hemisphere. But removing of 182,500 km3 of water per year from the Arctic Ocean and putting this volume of water to the Bering Sea would multiply this effect with unpredictable consequences for climate change not only in the Arctic Ocean but also in the Bering Sea (cooling and much more ice formation) and globally.
This project was criticized and improved by engineer Eugene Pastors. I will discuss his project in my next journal.


Comments