Soap, DOC, and Foamy Streams
Toolik Field Station, North Slope, AK
June 15, 2019
Video of the Day:
Filtering raw leachate (previously coarsely filtered) through a 0.2 micron filter, which served to remove finer particulate matter and microorganisms. As it passed through this filter, the appearance of the leachate changed quite markedly - from chocolate milk to light yellow in color, and from opaque to transparent.
The above video depicts the 0.2 micron filtration of the permafrost leachate. There’s much more on how this fits into the overall photo-bio experiment in my last post. This post specifically concerns the later portion of the video (starting at 20 seconds in), in which bubbles are visualized forming at the surface of the filtered leachate. I asked Dr. Cory about this, and the explanation is really interesting.
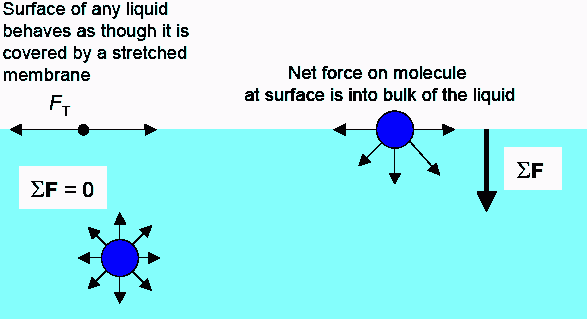
Let’s first talk about why bubbles form when you add soap to water and disturb the liquid. Pure water has a very high surface tension, which is a result of the large cohesive force between water molecules. This high surface tension tends to pull surface water molecules down toward the rest of the liquid, which makes it harder for bubbles to form.
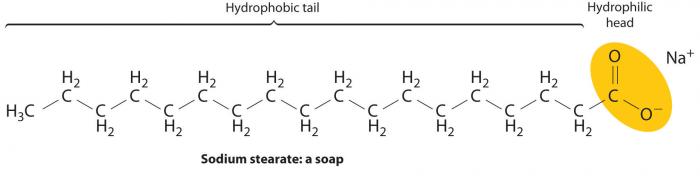
As the below diagram illustrates, soap molecules are amphipathic, in that they have both a polar end (hydrophilic, soluble in water) and a nonpolar end (hydrophobic, insoluble in water).
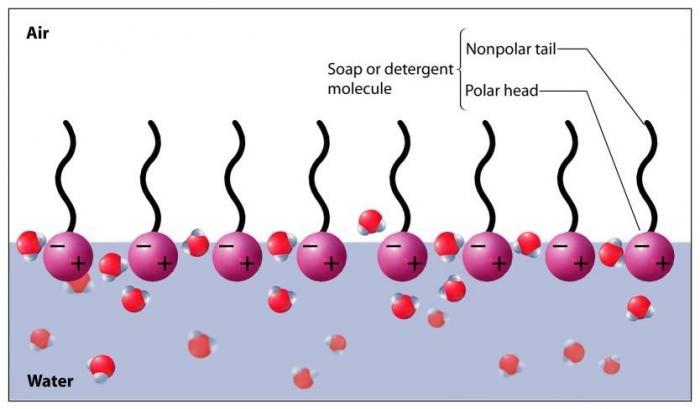
Soap dissolves in water, as the hydrophilic portions of the soap molecules (polar) form hydrogen bonds with the water molecules (polar). On the surface of the water, the presence of soap molecules serves to break up the hydrogen bonds between water molecules, leading to less cohesive force and decreased surface tension. This decreased surface tension makes it much easier for bubbles to form, and they do when air is introduced into the system (via agitating the solution).
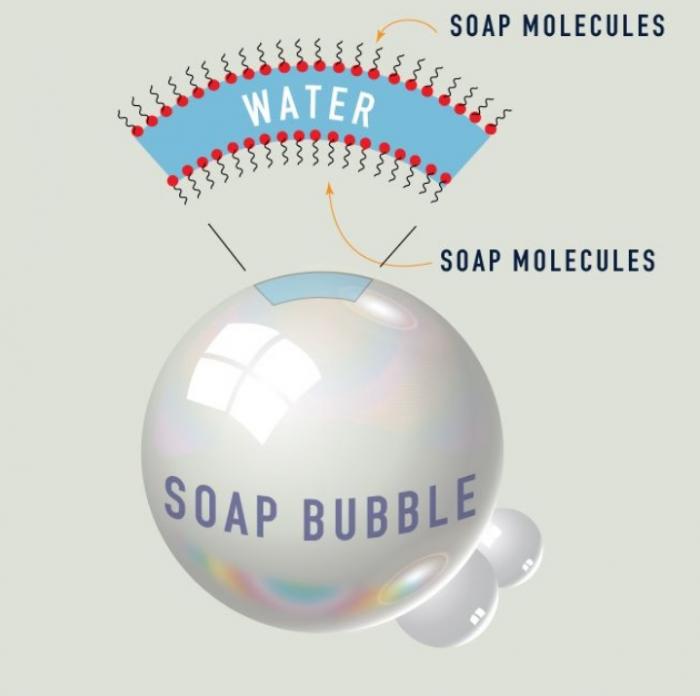
The below diagram illustrates the anatomy of a soap bubble, a very thin film of soapy water that serves to trap air.
So how does this relate to the bubbles we observed forming at the surface of the filtered permafrost leachate? The filtered leachate notably contains a high concentration of dissolved organic carbon (DOC). The composition of DOC is very complicated; check out this previous post of you need a refresher. The secret to the filtered leachate bubbles is that many of the constituents of DOC are amphipathic - they have polar (hydrophilic) and nonpolar (hydrophobic) regions. This allows these molecules to behave exactly like soap molecules - dissolve in the water, disturb the surface tension, and make it easier for bubbles to form.
You’ve probably noticed foam in streams before; this is the exact same process at work. As Dr. Cory pointed out, stream foam is associated with streams that have a very high DOC content. Makes sense!

Comment below!


Comments