I stuffed the cups with some sturdy brown paper towels to keep them separate and then placed them in a mesh laundry bag.

The Marine Scientist Technicans (MSTs) connected them to the CDT sampler that was dropped below 3300 meters!
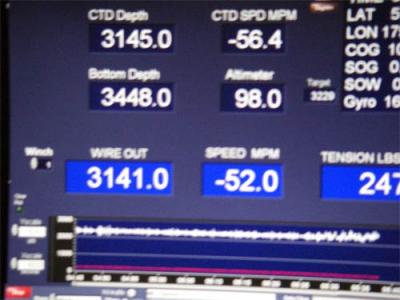
How much pressure was down there? Scott Hiller, from Scripps Oceanographic Institution, plugged some numbers into an equation and told me that there was some 5100 psi (pounds per square inch) acting on those little white cups. The temperature was just above freezing.


Two hours after dropping them down to the bottom of the Bering Sea, they emerged strapped and dripping.
And MUCH smaller.
Oh how CUTE!

So what did we learn from this?

Well, there are lots more questions that arise. How far do the cups have to drop in order for them to compress? What is the tipping depth, the depth that they begin to compress? Does the length of time that they are submerged make a difference in how they compress? Where does the gas that is in the cup go?
Ah, science, sweet science, raising more questions than answering once again.


Comments