Yesterday I attempted to explain why some soil microbes in wetland areas produce methane gas. In anaerobic conditions, carbon dioxide may be the only electron acceptor available. When this is the case, the soil microbes that can use CO2 in the oxidation/reduction process will produce methane as a byproduct of the process.
Today I will focus on the 'how' of this gas production. Buckle your seat belts for some more chemistry…
Metabolism and Cellular Respiration
In order for an organism to stay alive, chemical process occur within the organism. Collectively these processes are known as metabolic processes, or referred to as metabolism. These chemical processes involve making compounds (proteins, carbohydrates, fats) that build tissue, and storing energy in the bonds of sugar molecules. This is known as constructive metabolism.
Destructive metabolism is the breaking down of the sugars to produce usable energy and waste. Destructive metabolism is also known as cellular respiration.
Where does an organism get the sugars that are broken down in cellular respiration?
Remember that most plants make their own food from carbon dioxide, water, and sunlight. This is called photosynthesis, and the products are sugar and oxygen:
6H2O + 6CO2 + sunlight ==> C6 H12 O6 + 6O2
This is an oxidation/reduction process that produces a gas byproduct. Water is oxidized (loses electrons), carbon dioxide is reduced (gains electrons) to produce the sugar, with oxygen as a gas byproduct.
Plants are autotrophs: they make and metabolize their own sugars. The Petsikko Wetlands are surrounded by flowers at the moment, testimony to the plant's sugar-making photosynthetic process at work.


Organisms that cannot make their own sugars are called heterotrophs. They must get their sugars from other organisms. In other words, they have to eat to survive. Soil microbes are heterotrophs. As noted previously, soil microbes "feed" off dead organic matter in the soil.
Cellular Respiration in Operation
But how is the energy converted from stored energy in the bonds of sugar molecules into energy that an organism's cells can use to carry on life processes?
Cellular respiration is, you guessed it, another oxidation/reduction process. For aerobic respiration, the process is:
C6 H12 O6 + 6O2 ==> 6CO2 + 6H2 O
The sugar is oxidized (loses electrons), oxygen is reduced (gains electrons), to produce water, with carbon dioxide as a gas byproduct.
However, this chemical equation is only good where oxygen is available to be the electron acceptor. Remember, soil microbes live in anaerobic conditions (no oxygen available), and the oxidation/reduction process they use depends on what electron acceptor is available to them. I will add the redox table I made here again, just as a refresher.
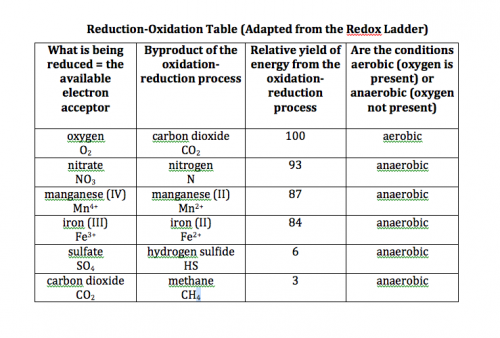
Methane-producing soil bacteria, at the bottom of the table, use carbon dioxide as the electron acceptor in cellular respiration.
Where Cellular Respiration Occurs:
Humans are multicellular organisms. Within each of our cells, there are specialized parts (called organelles). One type of organelle, the mitochondria, is known as the "power plant" of a eukaryotic cell (cells that have a nucleus and other organelles). It is inside the mitochondria that cellular respiration takes place.
You need a membrane (a boundary) to convert stored energy into usable energy. Mitochondria are filled with membrane. Look closely at this image of mitochondria inside a kidney cell. The wavy appearance inside them is membrane.
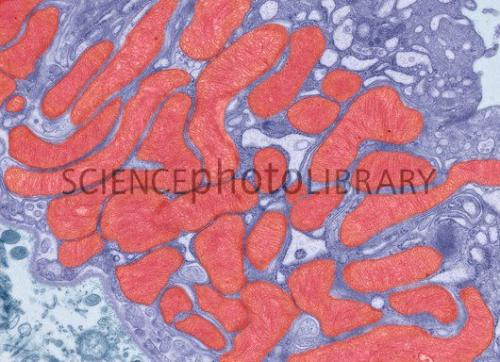
However, most soil microbes, including methane-producing bacteria, are single-celled organisms. They are prokaryotes, meaning they have no nucleus or organelles. So for single-celled soil microbes, it is the membrane of the single cell itself that is used during the cellular respiration process.
What Happens at the Membrane
The sugar fuels the proteins embedded within the membrane. These proteins pump protons (hydrogen ions, H+) across the membrane. However, the addition of protons to one side of the membrane causes an imbalance. When you have an increased number of protons in a solution, you have an acidic condition. The protein pump has caused an acid-base gradient across the membrane: higher acidity on one side than on the other.
Balance needs to be restored. Protons will pass back through the membrane, triggering a different protein in the membrane to create the ATP (adenosine triphosphate) energy molecule. It is the ATP energy molecule that is used by the cell for life processes.
To Review…
Energy stored in the bonds of sugar molecules causes a protein in a membrane to actively pump protons across the membrane. This movement of protons across the membrane causes an acid-base imbalance, so the protons move back across the membrane. The protons moving back across the membrane cause a different protein to trigger the creation of the ATP energy molecule.
So Part 1 + Part 2…
Now we know the 'hows' and the 'whys' of methane production by soil microbes in a wetland. Soil microbes live in anaerobic conditions. To survive, they need to turn the stored energy that they gain from breaking down dead organic material into ATP energy that they can use to support themselves. They do this through the oxidation/reduction process of cellular respiration, in which protons are moving back and forth across the cell membrane. This process requires electron acceptors, and under the more extreme anaerobic conditions where carbon dioxide is the only electron acceptor available, methane gas will be produced as a byproduct.

Congratulations – you made it through a lot of chemistry with some biology thrown in (after all, they are connected!).
But so what??
Well, remember methane is a very powerful greenhouse gas. It can trap 25 times more heat than carbon dioxide. The Arctic – the circumpolar north - has a lot of wetland area. The observational data that we collect in the Petsikko wetland is the part of Kim's work that is helping to quantify how much methane is produced by different types of northern wetlands.
However, Kim will also be running an experiment in the wetland later in the summer. Her question: If you can change the availability of electron acceptors in the wetland, can you change the activity levels of the various soil microbes so that methane production will be reduced?
Additional Reading on Redox:
Kim pointed me to this website to help me understand the sequence of oxidation-reduction reactions. She also recommended this website for further explaining redox reactions.


Comments