Photo-Bio Project Overview
Toolik Field Station, North Slope, AK
June 6, 2019
Video of the Day:
The Lapland longspur (Calcarius lapponicus) breeds in the summer on the tundra of the North Slope. The breeding male is quite distinctive, with black forehead and throat, white stripe above the eye, and a chestnut color on the nape of the neck.
At the outset of my stint up here at Toolik Field Station, I wanted to provide a general experimental overview of the summer photo-bio project being conducted by the Cory, Kling, and Crump labs. In future posts, I will review more specific details.
Relevant Background
As discussed previously, dissolved organic carbon (DOC) chemistry is very important in Arctic regions, as water sources are unshaded, usually shallower, and concentrated with DOC from permafrost melt. DOC can be partially oxidized or completely oxidized to CO2, and studies suggest that sunlight plays a greater role in this process than bacteria.1
DOC contains a wide variety of organic molecules. Here’s a previous post with lots more detail. Of these molecules, research suggests that aromatic compounds are more difficult for bacteria to break down, while aliphatic compounds are much easier (more labile).2
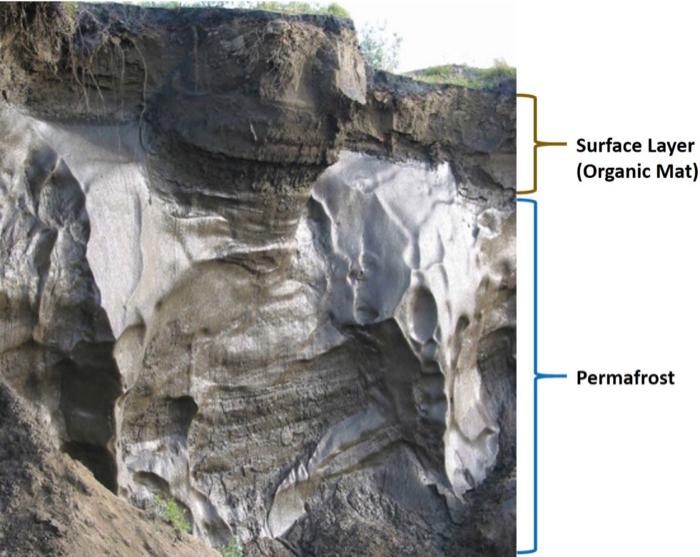
Studies have found that the general breakdown of organic content within Arctic soil differs based on depth. Surface (organic mat) layer soils in the Arctic tend to contain a greater concentration of larger, more oxidized, aromatic organics. Deeper permafrost, on the other hand, tends to contain higher prevalence of smaller, less oxidized, aliphatic compounds.3
Study Sites
The study sites for experiments this summer will be in the wet sedge tundra near an Arctic headwater stream. Wet sedge represents the dominant landscape of the lowland environment on the North Slope of Alaska.

Critical Question 1: How does DOC chemistry affect the metabolic process of microbes?
Addressing It
Soil pits will be excavated down to 1.2 meters at the study site. From each soil pit, samples will be taken from two categories of soil, based on depth: surface (organic mat) layer (10-30 cm in depth, proceeds through freeze-thaw annually) and permafrost (80-120 cm in depth, frozen for at least two years). Filter-sterilized DOC will by prepared from these soil samples via solid-liquid extraction (basically, preparing tea from soil) and subsequent filtration to remove all microbial content. This DOC will then be reinoculated with a set amount of microbial material from the same sample site and depth and incubated for seven days.
Microbial gene abundance and expression patterns will be evaluated through metagenomics and metatranscriptomics on samples taken at the beginning and end of the incubations. The goal here is to evaluate and quantify the microbial enzymatic reactions used to break down DOC.
To identify the specific formulas of the compounds in the DOC that are being consumed and produced during the incubations, mass spectrometry will be used. In addition, spectroscopy (Aqualog®) will be utilized to measure absorbance and fluorescence of the samples at different points.
Hypothesis
As compared to microbes in permafrost samples, microbes in samples taken from surface organic soils will exhibit greater expression of genes associated with more energy-consuming metabolism (e.g. breakdown of aromatic organic material) and will exhibit less efficient growth. The rationale for this hypothesis is that surface organic material houses compounds more difficult for bacteria to metabolize (see above).

Critical Question 2: How does DOC exposure to sunlight factor into this equation?
Addressing It
The incubation sequence above will be expanded to include two categories of filter-sterilized “DOC tea” - dark control (foil-wrapped) and sunlight-exposed (12-18 hours).
Hypothesis
As compared to microbes in dark control samples, microbes in sunlight-exposed samples will exhibit reduced expression of genes associated with enzymatic pathways that break down DOC. The rationale for this hypothesis is that sunlight has been shown to be very important in the breakdown of aromatic and oxidized DOC (see previous post).1 In other words, sunlight is doing quite a bit of work for the microbes.

Critical Question 3: How does long-term microbial community adaptation affect the rate of DOC breakdown?
Addressing It
A wide variety of data will be collected at the beginning and end of the incubation periods for both dark control and sunlight-exposed samples - abundance of microbes, CO2 production, O2 consumption, and microbial community composition.
Hypotheses
For samples taken from permafrost, exposure to sunlight will result in higher microbial growth efficiency, an increase in microbial uptake, and a species composition shift toward microbes more adapted to process aliphatic compounds, over the seven day incubation period. The rationale here is that:
- PermafrostPermanently frozen ground. contains a greater concentration of smaller, aliphatic, less oxidized compounds, so bacteria in permafrost have specialized to metabolize this compound class.
- Sunlight serves to break down large, highly oxidized aromatic compounds into smaller aliphatics. In other words, sunlight helps produce more fuel for the majority of bacteria in permafrost already equipped to metabolize it.
For samples taken from surface layer (organic mat) soils, exposure to sunlight will result in lower microbial growth efficiency, a decrease in microbial uptake, and a species composition shift toward microbes more adapted to process aliphatic compounds, over the seven day incubation period. The rationale here is that:
- The surface mat layer of soil contains a greater concentration of larger, aromatic, highly oxidized compounds, so bacteria in these soils have specialized to metabolize this compound class.
- Sunlight serves to break down large, highly oxidized aromatic compounds into smaller aliphatics. In other words, sunlight is breaking down (removing) the fuel for the majority of bacteria in surface layer soils.

Why Does This Matter?
As previously discussed, a large amount of CO2 is being produced from DOC produced by thawing permafrost (check out this post for more). It is of utmost important to understand the specifics of how DOC is being broken down to CO2 in Arctic watersheds in order to effectively update climate models that serve to predict future CO2 levels. Our current understanding of the factors that control DOC breakdown in the Arctic is very limited. This work will serve to elucidate the fundamental interplay between sunlight and bacteria in DOC breakdown.
Comment below!
-
Cory, R. M., et al. “Sunlight Controls Water ColumnHypothetical 'cylinder' of water between the surface of the ocean and the ocean bottom. Processing of Carbon in Arctic Fresh Waters.” Science, vol. 345, no. 6199, 2014, pp. 925–928. ↩︎ ↩︎
-
Vallino, J. J., et al. “Modeling Bacterial Utilization of Dissolved Organic MatterMaterials and debris that originated as living plants or animals.: Optimization Replaces Monod Growth Kinetics.” Limnology and Oceanography, vol. 41, no. 8, 1996, pp. 1591–1609. ↩︎
-
Ward, Collin P., and Rose M. Cory. “Chemical Composition of Dissolved Organic MatterMaterials and debris that originated as living plants or animals. Draining PermafrostPermanently frozen ground. Soils.” Geochimica Et Cosmochimica Acta, vol. 167, 2015, pp. 63–79. ↩︎


Comments