Closer Look: Dissolved Organic CarbonOrganic carbon compounds form the physical basis for all living organisms.
Austin, Texas
May 25, 2019
Photo of the Day:

As covered in my previous two posts on permafrost and the permafrost positive feedback loop, a great deal of organic material is being unlocked from thawing permafrost and remobilized. I wanted to learn more about this organic material.
What Is It?
Organic material in solution in rivers and streams is called Dissolved Organic Carbon, commonly abbreviated DOC. Being the product of decomposition of dead plants, animals, and microbes, it has a very complex makeup, one which is not comprehensively understood.1 As DOC composition varies geographically, I will focus specifically on Arctic DOC here. Mass spectrometry has indicated that Arctic DOC contains lignins, condensed aromatics, lipids, tannins, unsaturated hydrocarbons, peptides, carbohydrates, and amino sugars, along with many other aliphatics.2
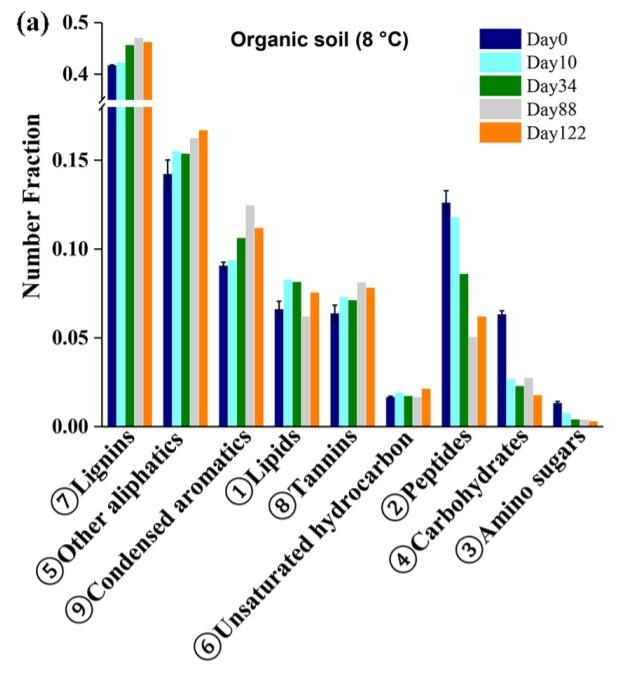
DOC Compound Classes
Let’s define these compound classes in more detail.
- Aromatics: Organic molecules that contain planar ring structures with exceptional stability due to a high degree of electron delocalization.
- Aliphatics: Organic compounds that are non-aromatic.
- Lipids: Fatty acids or their derivatives.
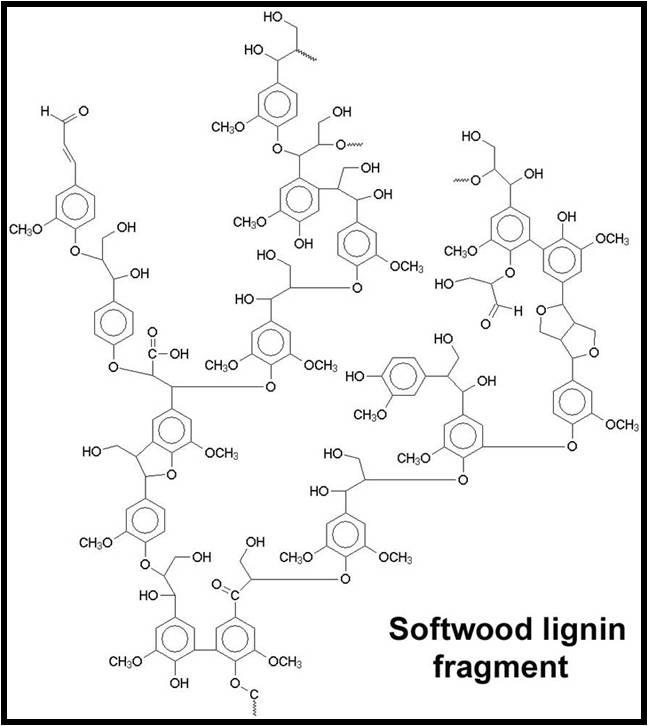
- Lignins: A class of organic polymers present in the structural tissue of plants. The commonality of lignins in DOC makes sense, as these polymers are extremely abundant in terrestrial plant material and very resistant to biodegradation.34
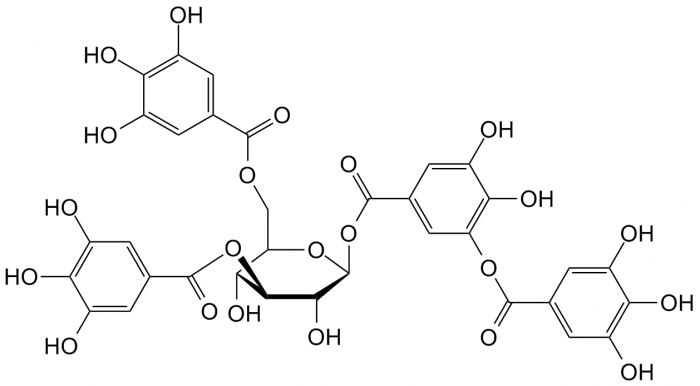
- Tannins: Complex biomolecules present in plant tissue that serve to bind and precipitate proteins. They play a role in plant defense against predation (as they give tissue a characteristic astringency) and are also involved in control of plant growth.4
- Unsaturated Hydrocarbons: Organic compounds that contain pi bonds and/or rings.
- Peptides: Amino acid chains linked by peptide bonds.
- Carbohydrates: Sugars, starch, or cellulose
- Amino Sugars: Sugars in which one of the hydroxyl (-OH) groups has been replaced by an amine group (-NH2, -NHR, -NR2).
Why Is It Colorful?
As alluded to in my previous post, DOC often makes Arctic streams quite colorful (brown, yellow, even sometimes red). Where does the color come from?
Electrons within organic molecules form waves called molecular orbitals. When a compound absorbs energy, electrons are promoted (excited) to higher energy molecular orbitals. For most organic molecules, the absorbed energy is within the ultraviolet region of the spectrum. Accordingly, most organic molecules absorb ultraviolet light, reflect the entire visible spectrum, and appear white as a result.
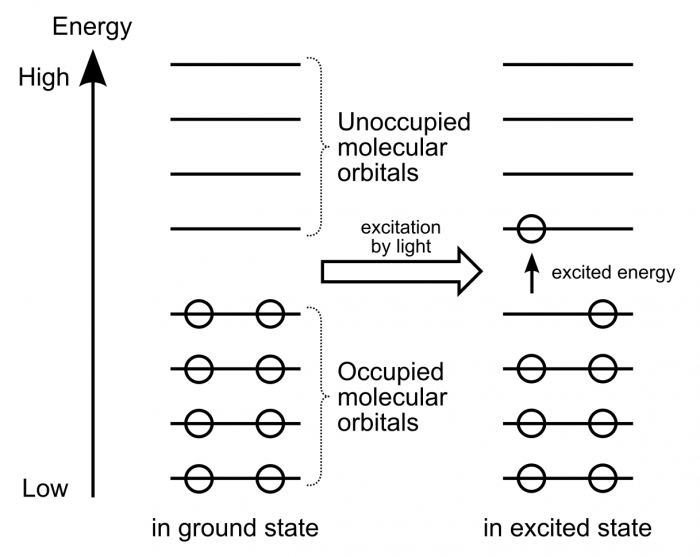
Compounds with higher degrees of electron delocalization have more overlapping regions of electron density. As a result, these compounds have more molecular orbitals. These molecular orbitals are more similar to each other in energy, and less energy is required to promote electrons to higher energy molecular orbitals.
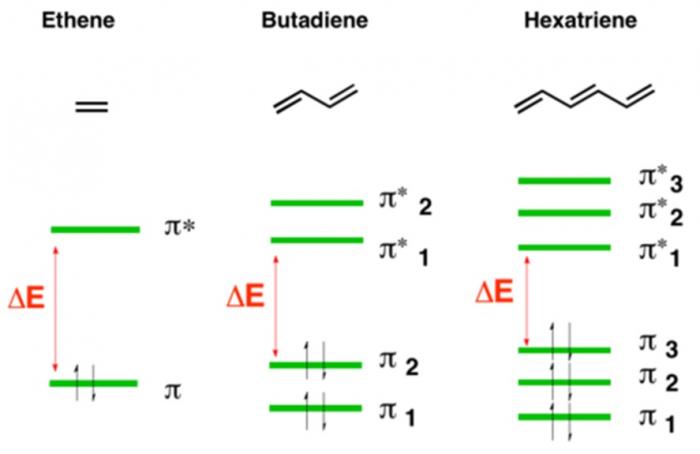
So, compounds with more electron delocalization absorb less energetic light. It follows that if a compound has enough electron delocalization, the energy absorbed is no longer in the ultraviolet range, but rather in the visible range. If a compound absorbs energy in the visible range, it no longer reflects that entire range back to the viewer. Instead of appearing white, it now appears colored!
Take a look at the amount of electron delocalization in the above lignin and tannin examples. It makes complete sense that these compounds are colorful.

Other Examples
Here are some other neat examples of this same concept.
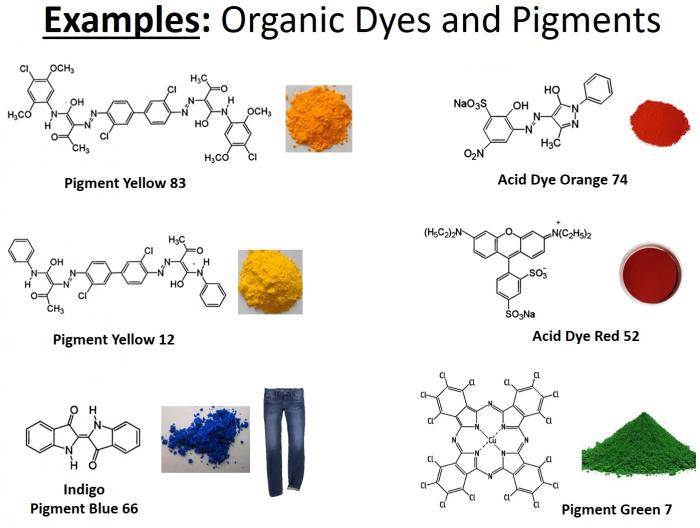
- Below is a graphic illustrating some of the more common organic dyes and pigments. Note the degree of electron delocalization in these compounds.
- Below is a great graphic prepared by Compound Interest illustrating the colorful compounds present in highlighters. Again, note the degree of electron delocalization in these compounds.

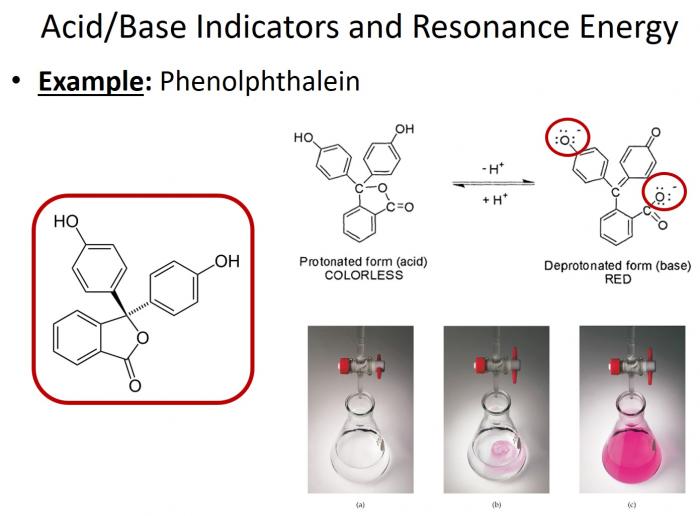
- One of the most commonly used acid-base indicators in phenolphthalein. This compound notably changes colors based on pH. In acidic conditions, it appears clear; in basic conditions, it appears pink. But why? The same concept applies here! The degree of electron delocalization in this compound changes based on whether it is protonated (acidic conditions) or deprotonated (basic conditions). Only in its deprotonated state is the electron delocalization great enough to allow for absorption in the visible spectrum (and accordingly, pink color!).
If you're interested, here's a presentation I use in my organic chemistry course with even more information on electron delocalization and color in organic chemistry:
Comment below!
-
Hawkes, Jeffrey A., et al. “Extreme Isomeric Complexity of Dissolved Organic MatterMaterials and debris that originated as living plants or animals. Found across Aquatic Environments.” Limnology and Oceanography Letters, John Wiley & Sons, Ltd, 15 Feb. 2018, aslopubs.onlinelibrary.wiley.com/doi/full/10.1002/lol2.10064. ↩︎
-
Chen, Hongmei, et al. “Molecular Insights into Arctic Soil Organic MatterMaterials and debris that originated as living plants or animals. Degradation under Warming.” Environmental Science & Technology, vol. 52, no. 8, 2018, pp. 4555–4564. ↩︎
-
Hillel, Daniel, and Jerry L. Hatfield. Encyclopedia of Soils in the Environment. Elsevier/Academic, 2005. ↩︎
-
Maier, Raina M., et al. Environmental Microbiology. Academic Press, 2000. ↩︎ ↩︎


Comments