Arctic Oxidation Culprits
Austin, Texas
May 26, 2019
Photo of the Day:

PermafrostPermanently frozen ground. houses around 1.6 billion tons of carbon that could potentially be released as dissolved organic carbon (DOC) as the climate warms.1 As noted in my previous post on the permafrost positive feedback loop, this unlocked organic material is being converted in carbon dioxide and serving to intensify global warming, especially in Arctic regions. In this post, I’d like to focus on the specific mechanisms by which DOC is oxidized to carbon dioxide.
What Is Oxidation?
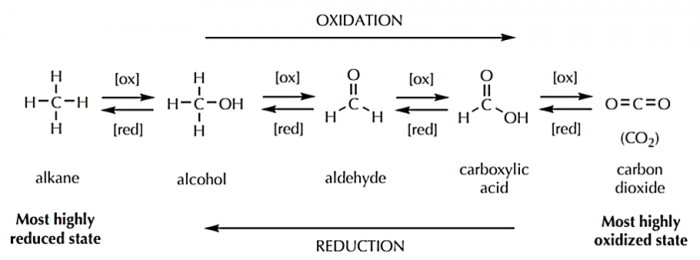
You might remember the mnemonic from your high school chemistry class: LEO the lion says GER. LEO stands for Lose Electrons Oxidation; GER stands for Gain Electrons Reduction. In a chemical reaction, carbon is oxidized when it loses electron density, and it is reduced when it gains electron density.
Organic chemists denote oxidation reactions as those in which carbon gains one or more bonds to a more electronegative element (typically oxygen in organic reactions). In doing so, carbon is losing electron density. Reductions are accordingly defined as reactions in which carbon gains one or more bonds to a less electronegative element (typically hydrogen in organic reactions).
As you can see in the below diagram, when carbon is completely oxidized, the end product is carbon dioxide (in which all four of carbon's bonds are to oxygen).
Bacterial Decomposition and Respiration
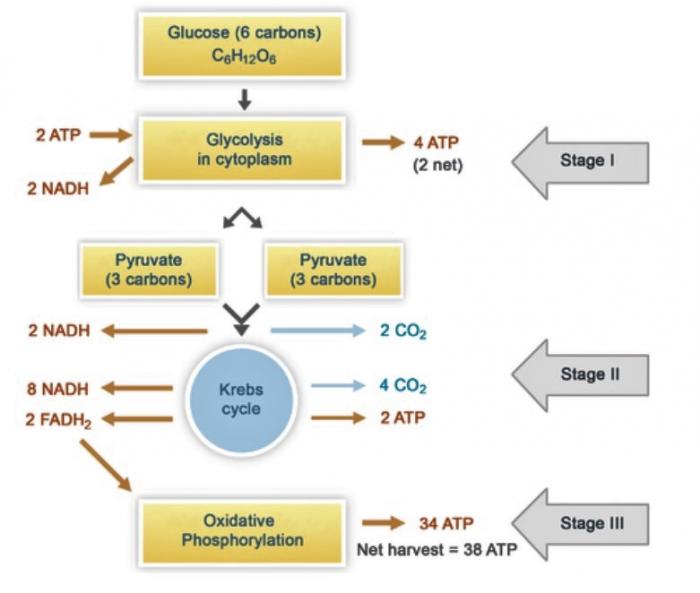
One of the possible mechanisms by which DOC is oxidized to carbon dioxide in Arctic watersheds is bacterial decomposition and respiration. In decomposition, the complex organic molecules present in DOC (lignins, aromatics, etc.) are broken down by bacterial enzymes.2 This process yields many nutrients, one of which is glucose, the fuel of cellular respiration. In aerobic cellular respiration, glucose (C6H12O6) and oxygen are converted into carbon dioxide, water, and energy.
Overall Reaction for Aerobic Respiration:
C6H12O6 + 6O2 → 6CO2 + 6H2O + Energy
This overall reaction can be broken down into three stages: glycolysis, the Krebs cycle, and oxidative phosphorylation. In glycolysis, glucose (6 carbons) is broken down into two molecules of pyruvate (3 carbons each). In the Krebs cycle, each pyruvate is oxidized into three molecules of carbon dioxide. Oxidative phosphorylation is the terminal metabolic pathway in aerobic respiration, in which byproducts of the Krebs cycle (NADH and succinate) are oxidized to generate energy.
Photooxidation
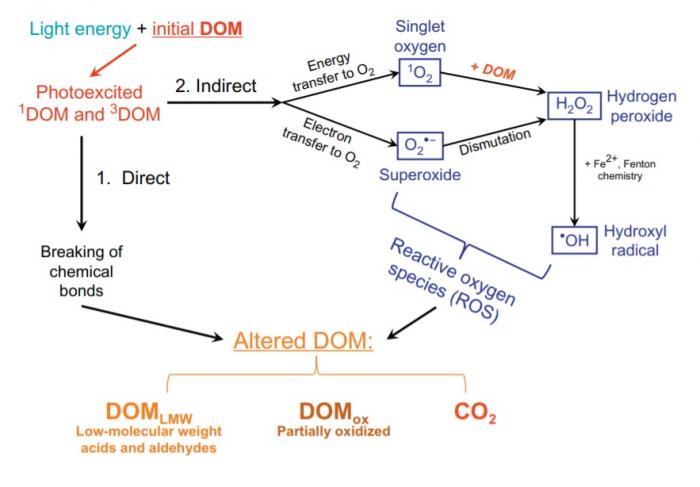
Photooxidation from sunlight exposure represents a second pathway by which DOC is oxidized to carbon dioxide. As mentioned in my previous post, many of the constituents of DOC have a high degree of electron delocalization. Accordingly, DOC is able to absorb a wide breadth of energy from sunlight, both in the ultraviolet and visible range. When a component of DOC absorbs energy from the sun, electrons in the molecule are promoted to higher energy molecular orbitals, and the molecule adopts an excited state. At this point, there are two potential outcomes:
- It is broken down directly into simpler constituents.3
- It reacts with water, oxygen, and iron in the water to yield peroxides, superoxide, other reactive oxygen species, and oxygen radicals. Here, a critical reaction is the photo-Fenton reaction, in which iron reacts with hydrogen peroxide to yield oxygen radicals. Reactive oxygen species are in turn responsible for partially or completely oxidizing DOC, even DOC not originally susceptible to direct photochemical degradation.3
The Gist of It All
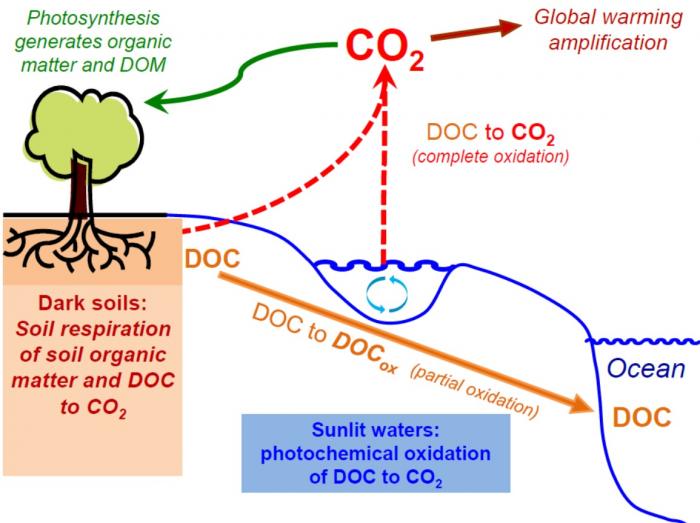
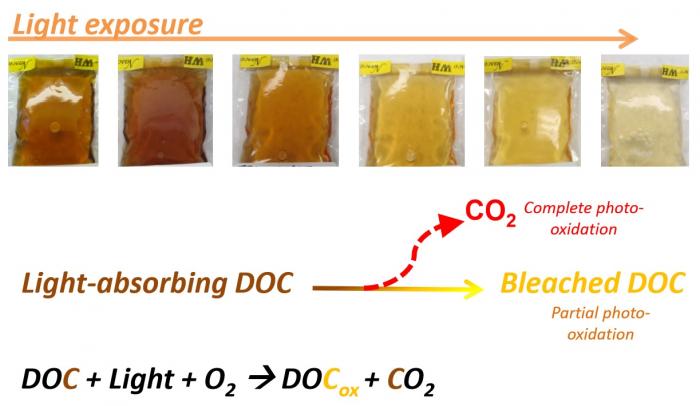
So, two general processes are responsible for oxidation of DOC in Arctic watersheds: bacterial degradation/respiration and photooxidation from sunlight exposure. Below is a great summary graphic.
Arctic Fact of the Day:
Recall that it is the high degree of electron delocalization in many components of DOC that allows these compounds to absorb light in the visible spectrum, yielding colorful Arctic streams. When DOC is broken down by sunlight, networks of electron delocalization are broken, and the water becomes lighter and lighter. This is called photobleaching.1
Arctic Question of the Day:
Which is the more important factor controlling oxidation of DOC in Arctic watersheds: bacterial degradation/respiration or photooxidation from sunlight?
(Comment below!)
-
Taterka, Bruce and Rose Cory. “Thawing PermafrostPermanently frozen ground. Lessons and Lab Manual: A Multi-Lesson Resource.” PolarTREC, 20 Sept. 2015, www.polartrec.com/resources/lesson/thawing-permafrost-lessons-and-lab-manual-a-multi-lesson-resource. ↩︎ ↩︎
-
Kowalski, Kathiann. “Recycling the Dead.” Science News for Students, 6 Aug. 2016, www.sciencenewsforstudents.org/article/recycling-dead. ↩︎
-
Kaplan, L.A., and R.M. Cory. “Dissolved Organic MatterMaterials and debris that originated as living plants or animals. in Stream Ecosystems: Forms, Functions, and Fluxes of Watershed Tea.” Stream Ecosystems in a Changing Environment, 1st ed., Academic Press, 2016, pp. 241–320. ↩︎ ↩︎


Comments